The magnitude of epidemic has prompted several drugs and new treatment as we entered the phases of contingency. Developing new drug is time consuming and the safety efficacy is paramount, thus there was the need to accelerate COVID-19 interventions that can work faster and effectively. Drug repurposing is the process to identify the new indications for existing drugs as an efficient and economical way. Currently, existing antivirals and combination therapy are recommended to attain significant clinical benefit. Since the outbreak began, a massive influx of computational papers identifying possible antiviral drug repurposing candidates have been published in both peer-reviewed and preprint servers with and without confirmatory studies. Repurposing can be the most straight forward way to deliver and will continue to play an important role in pharmacological treatment accompanied with funding, collaboration, and openly publishing raw research data to allow for data mining and pooling of trials. In spite of IP waiver proposal made in Oct 2020, greatest number of COVID patent filings were reflected related to conventional vaccine technologies and repurposed drugs, followed by more-novel vaccine technologies like mRNA.
Repurposed drugs Patent data
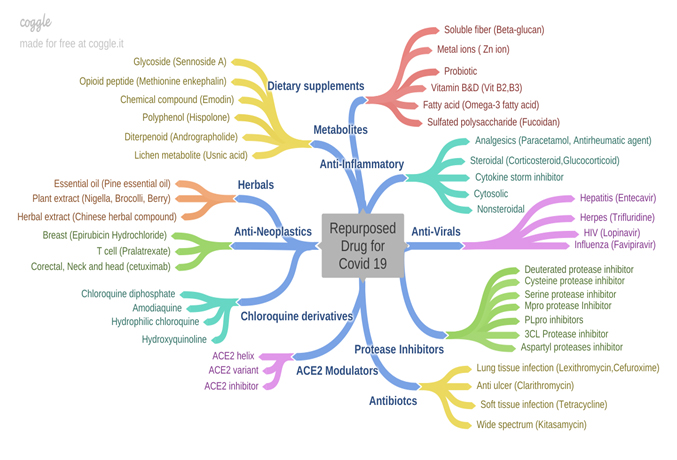
Computational Drugs
-
Azilsartan
-
Cabergoline
-
Candisartan Cilexetil
-
Compound
-
Dipiveffin
-
Dipivefrin
-
Domperidone
-
Droperidol
-
Fluvastatin
-
Imatinib
-
Irbesartan
-
Losartan
-
Lovastatin
-
Olmesartan
-
Telmisartan
-
Valsartan
Jurisdiction (Patents)
Loading..........
The Data is Not Available
Patent Publication Trend for Repurposed drug for patent
No Data Found
Assignee Having both Patent and Clinical trial
Yale University
King Abdulaziz University
University of Chicago
Most invested Repurposed drug for Clinical trials
Anti-Viral
Remdesivir
Ritonavir
Anti-Inflammatory
Dexamethasone
Ivermectin
Chloroquine derivative
Hydroxychloroquine
Chloroquine phosphate
Antibiotic
Doxycycline
Tetracycline
Anti-Neoplastic
Acalabrutinib
Duvelisib
Kinase Inhibitor
Baricitinib