CAR-T Cell Therapy
Cancer is a condition in which cells undergo unrestricted growth due to variations in DNA. Cancer is one of the diseases that can affect any part of the body. There is a wide range of factors that could lead to cancer development such as lifestyle, food, medications, infections, chemicals, heredity, hormones, etc. Next comes the question of whether any treatment is available with a complete cure. Extensively studied and FDA-approved therapies are available for various types of cancer.
Well-known types of cancer therapies used across the world as treatments
Some of the well-known therapies for the treatment of cancer are:
- Chemotherapy
- Hormone therapy
- Hyperthermia
- Photodynamic therapy
- Radiation therapy – Radiation therapies can be divided into the following three groups broadly:
- External beam radiation therapy (EBRT or XRT) or teletherapy;
- Brachytherapy or sealed source radiation therapy; and
- Systemic radioisotope therapy or unsealed source radiotherapy.
- Interventional radiology – This technique comprises of medical imaging methods for leading the cancer treatment. A few types of treatments involve:
- Ablation cancer therapy – RFA Therapy
- Microwave ablation (MWA)
- Cryoablation
- Percutaneous ethanol ablation (PEI)
- Stem cell transplant
- Surgery
- Palliative care
- Laser therapy
- Immunotherapy – This type of treatment involves improvement in immune system. Some of immunotherapy treatments are:
- Immune checkpoint inhibitors
- Cell therapy
- CAR T-cell Therapy
- CAR NK cell therapy
- TCR T-cell Therapy
- Monoclonal Antibodies
- Immune system Modulators
- Gene editing
- Personalized medicine
Recent advancements in cancer therapy - Immunotherapy
Immunotherapy treatments are those that help strengthen the immune system and combat cancer. Immunotherapy based cancer treatments have proven effective against various malignant cancers, such as breast, cervical, head, kidney, and neck cancers, B-cell lymphoma, Hodgkin lymphoma, non-Hodgkin lymphoma, melanoma, multiple myeloma, and more. Monoclonal antibodies, such as rituximab, cetuximab, and nivolumab, are used to activate the immune system and treat these cancers. Another emerging immunotherapy treatment is cell therapy, in which infected cells can be replaced with healthy ones.
CAR -T Cell Therapy
Chimeric antigen receptor (CAR) T-cell therapy is an immunotherapy treatment that uses a patient’s genetically modified T-cells for the recognition and attack of cancer cells. CAR T-cell therapy is more effective against certain types of cancers, such as B-cell leukemia, and a response rate of 50% was observed in patients with relapsed (cancer recurrence after initial treatment) or refractory (lack of response to other treatments) diffuse large B-cell lymphoma. There are still challenges to overcome in the development and implementation of CAR T-cell therapy, such as cytokine release syndrome (CRS), which is a side effect of immunotherapy treatment where a large amount of cytokine proteins are released, leading to inflammation in the body. Additionally, neurotoxicity can occur, which can affect the function of the nervous system. Resolving these side effects requires personalized therapies.
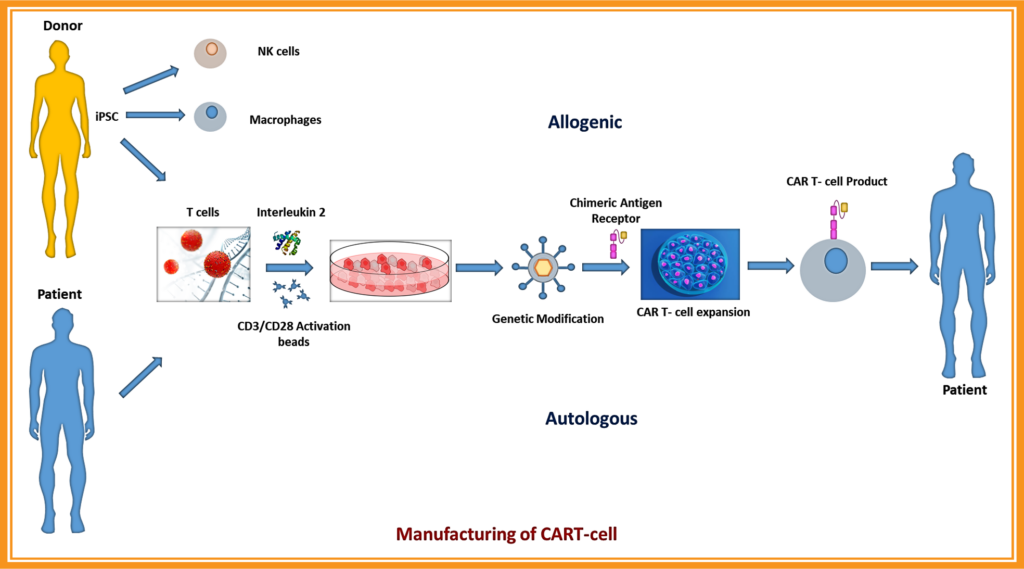
Manufacturing of CAR-T cell
Chimeric antigen receptor (CAR) T-cell therapy involves targeting specific tumor markers, such as CD19, MUC16, MUC1, CA1X, CEA, CD8, CD7, CD10, CD20, CD22, etc., to identify cancerous cells. CAR- T cell therapy is known for its process, which includes isolating T cells (a type of white blood cell) from the patient, genetically modifying the cells, multiplying them, and transferring them back to the patient, where they attack cancer cells. In the case of allogeneic cell therapy, T cells from a donor are used. Many companies and university research and development departments are working towards developing cancer therapies. Recently, the FDA approved the following CAR-T cell therapies:
- Kymriah (tisagenlecleucel) – Approved in 2017, obtained by Novartis Pharmaceuticals Corp for treating B-cell precursor acute lymphoblastic leukaemia (ALL), DLBCL, and follicular lymphoma (FL).
- Abecma (idecabtagene vicleucel) – Approved in 2021, obtained by Bristol-Myers Squibb Company (Celgene Corporation) for treating Multiple Myeloma.
- Tecartus (brexucabtagene autoleucel) – Approved in 2021, obtained by Gilead Sciences (Kite Pharma) for treating mantle cell lymphoma or ALL.
- Yescarta (axicabtagene ciloleucel) – Approved in 2021, obtained by Gilead Sciences (Kite Pharma) for treating Large B-cell lymphoma, FL.
- Carvykti (ciltacabtagene autoleucel) – Approved in 2022, obtained by Johnson & Johnson’s and GenScript Biotech Corporation for treating BCMA.
- Breyanzi (lisocabtagene maraleucel) – Approved in 2022, obtained by Bristol-Myers Squibb Company (Juno Therapeutics, Inc.) for treating large B-cell lymphoma.
What is the Intellectual Property factor in CAR-T cell therapy?
After the approval of CAR-T cell therapy by the FDA, approximately 65 companies are involved in CAR-T cell therapies for various types of cancer treatment. Thirty-five percent of the companies are in the prototype stage of CAR-T cell development for cancers targeting antigens.
Key players in the market who deals with CART-cell production and involved in CART-cell therapies are NOVARTIS, GILEAD SCIENCES, JOHNSON & JOHNSON, GENSCRIPT BIOTEC CORPORATION, PROMAB BIOTECHNOLOGIES, BIO4T2, ORICELL THERAPEUTICS, CARSGEN THERAPEUTICS, ALLOGENE THERAPEUTICS, BRISTOL-MYERS SQUIBB CO, ELPIS BIOPHARMACEUTICALS, KIROMIC BIOPHARMA, T MAXIMUM BIOTECH, AUTOLUS THERAPEUTICS, CELLECTIS, CHONGQING PRECISION BIOTECH, CTG PHARMA, ELICERA THERAPEUTICS, KAEDI BIOTHERAPEUTICS, and MUSTANG BIO.
What has been the trend of innovation based on studies related to CART-cell therapy?
Research related to CAR-T cell therapy started in the early 1990s, but research on CART-cell therapy for cancer treatment became extensive after 2014.
Patent innovation trend on CAR-T cell therapy
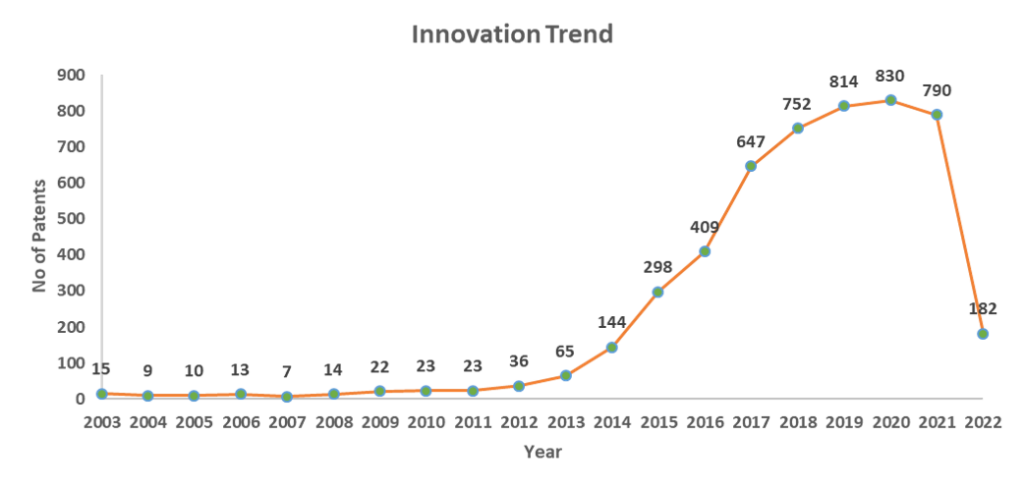
First developed and approved CAR T cell-based therapy was tisagenlecleucel (Kymriah) in 2017 for cancer patients until the age of 25. Since then, there has been significant research on CAR T cell therapy for various types of cancer.
Where all the patents are filed globally for CAR-T cell therapy?
Market coverage
No Data Found
Maximum number of patents are filed in China and European Countries in the field of CART-cell therapy. Around 20 CART-cell therapy companies are established in these two countries.
Key Players in the Domain of CAR-T cell Therapy
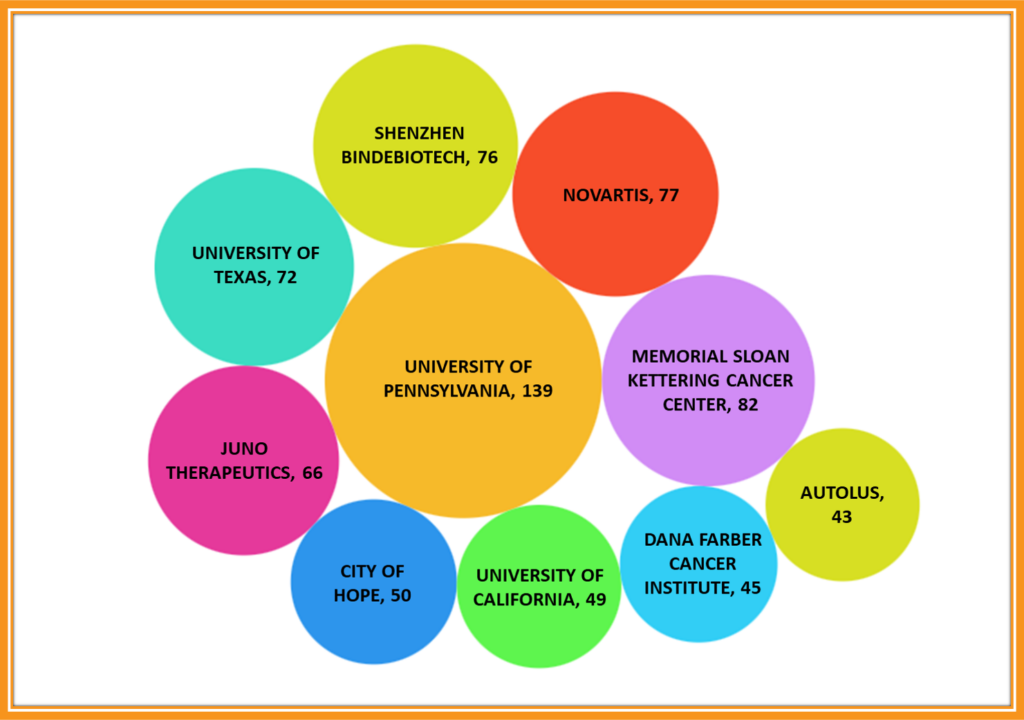
The University of Pennsylvania primarily focuses on the preparation of CAR-T cells using the autologous method. This means that immune cells taken from the patient themselves are used for the treatment.
Memorial Sloan Kettering Cancer Centre focuses on developing innovative approaches for diagnosing and treating different types of cancer using T-cells derived from patients.
Conclusion
Research on CAR-T cell therapy in cancer treatment is still ongoing, during which life-threatening side effects such as delayed immune cell reconstitution, nervous system toxicity, severe allergies, bleeding, and bone marrow infection need to be addressed. Because of this, patients need to remain in the hospital and be observed for a few more days. Therefore, efforts to reconstruct CAR-T cell therapy are being made to reduce the side effects caused by this treatment. CAR-T cell therapy is likely to be introduced in India by 2024. However, this therapy is not cost-efficient and has aftereffects. Therefore, extensive research is required in CAR-T cell therapy so that this advanced immunotherapy can be made more accessible and affordable for people suffering from cancer, who can be treated without any side effects. Some of the prominent future innovations for cancer treatment could include personalized vaccines, the use of the microbiome in treatment, and “smart” cancer drugs that specifically target tumor cells instead of healthy cells.
How can MCRPL help?
We at MCRPL help in conducting technology and competitive landscape studies on various technologies including buzzing topics such as CAR-T cell therapy. We are a team of 200+ patent and scientific literature experts and have performed technology intelligence/ scouting projects of more than 10,000 hours providing various actionable intelligence to our existing Fortune 500 clients.
© Molecular Connections Private Limited
For more information, contact priorart@molecularconnections.com
For more updates subscribe IP Tech Insider