The Food and Drug Administration (FDA)
The Food and Drug Administration (FDA) is responsible for the approval and regulation of medical devices, drugs, food items, vaccines, etc. This regulation is necessary to ensure the safety, reliability, and potency of these products. All kinds of medical devices and drugs, including those that require and do not require prescription, are approved by the FDA. Based on the approval level in the FDA, products can fall into categories such as:
- FDA-approved products, which are those that receive approval after ensuring that the benefits outweigh the risks.
- FDA-registered products are those about which the FDA is informed but cannot use the FDA logo.
- Finally, there are FDA-cleared products, which are comparable to existing products and have not undergone meticulous examination. The FDA is not responsible for the approval of animal vaccines, estrogen, multivitamins, or cosmetics, except for color additives.
Medical devices and FDA approval
The association working along with the FDA for approving medical devices is the Center for Devices and Radiological Health (CDRH). In most cases, medical devices require Premarket Approval (PMA) from the FDA before they can be sold in the market. This PMA guarantees the safety and effectiveness of the devices through scientifically proven evidence. The FDA has classified medical devices into three classes:
- Class I devices, such as bandages, stethoscopes, surgical masks, and manual wheelchairs, pose the least risk to individuals and do not require a PMA.
- Class II devices, such as automatic wheelchairs, pregnancy kits, syringes, and contact lenses, pose a moderate level of risk and require a 510(K) [clarifying the harmlessness and effectiveness] in the PMA process.
- Class III medical devices, such as implants, pacemakers, defibrillators, and stents, must meet the PMA requirements as they can pose a risk to individuals.
Chart showing the number of medical devices got approved from 2013-2023
No Data Found
* Till April 2023 data is considered for 2023
Chart showing the key technology areas and their medical devices approved from 2013 to 2023
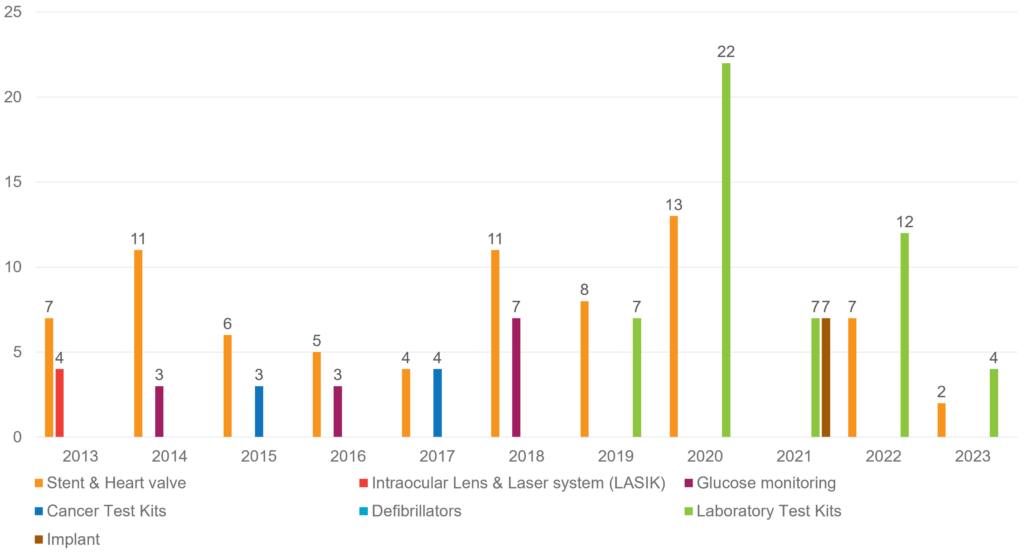
* Till April 2023 data is considered for 2023
Medical Device Approvals in 2023
Medical devices approved by the FDA from January 2023 to April 2023 are:
- 1 breathing stimulator
- 2 eye diagnostic devices
- 1 gel implant
- 1 glucose monitor
- 4 lab test kits
- 2 stimulators
- 1 spinal implant
- 2 stent devices
- 1 syringe applicator
- 1 haemorrhage blocker
Examples of these approved medical devices include the NeuRx Diaphragm Pacing System (NeuRx DPS) by Synapse Biomedical, Inc. in the US, Sculptra P030050/S039 by Q-Med AB in Sweden, and the CALCIVIS Imaging System – P170029 by CALCIVIS Limited in the UK.
FDA & Drugs Approval
The Center for Drug Evaluation and Research (CDER) is the division of the FDA responsible for approving prescription drugs in the United States. CDER ensures that drugs are safe and effective for their intended use. They review data provided by the manufacturer, conduct inspections and tests if necessary, and make a determination on whether to approve the drug. CDER also monitors drugs after they are on the market to ensure continued safety and effectiveness.
In emergency situations or for drugs that treat serious or life-threatening conditions, CDER may expedite the approval process. This is known as accelerated approval and allows the drug to be available to patients sooner.
Types of applications for FDA drug approval
There are several types of applications for FDA drug approval. The most common is the New Drug Application (NDA), which includes data on the drug’s safety, effectiveness, and manufacturing process. Another type of application is the Abbreviated New Drug Application (ANDA), which is used for generic drugs that are the same as a brand-name drug already on the market. The FDA also reviews applications for Over-the-Counter (OTC) drugs, Biologics License Application (BLA) for vaccines and other biological products, and Investigational New Drug (IND) applications for experimental drugs being studied in clinical trials.
Chart illustrating the number of drugs that got approved yearly from 2013-2023
No Data Found
* Till April 2023 data is considered for 2023
Drug Approvals in 2023
The list of drugs approved by FDA from January 2023 to April 2023 includes 7 Intravenous injection type drugs for example Leqembi, Posluma etc, 8 Oral tablet drugs like Brenzavvy, Jaypirca etc, Oral capsule drug Skyclarys, Nasal spray drug Zavzpret, Oral solution drug Daybue, Intrathecal injection type Qalsody, Topical ophthalmic solution form Miebo and Subcutaneous injection type Epkinly.
How MCRPL helps on Bio searches
MCRPL, a leading patent service provider, specialises in various searches and studies across various domains like biotechnology, gene editing and IP, medical devices with AI/ robotics applications, chemicals and drugs. We excel in providing tailor-made reports that fulfil customer needs and satisfaction based on our in-house tools and databases. Our expertise allows us to develop tailored strategies and craft robust patent claims that meet the evolving standards of patentability. Whether it’s conducting prior art searches, guiding patent prosecution, or drafting claims, our dedicated team is committed to helping inventors and businesses protect their innovative ideas and navigate the evolving landscape of patent eligibility. We stay up-to-date with the latest grant/publication processes, including publication alert updates, to provide our clients with the most effective strategies for protecting their intellectual property.
© Molecular Connections Private Limited
For more information, contact priorart@molecularconnections.com
For more updates subscribe IP Tech Insider